KINASE SELECTIVITY
CALQUENCE delivers focused kinase inhibition and limited off-target enzyme inhibition2,3
KINASE SELECTIVITY IN
PRECLINICAL MODELS3
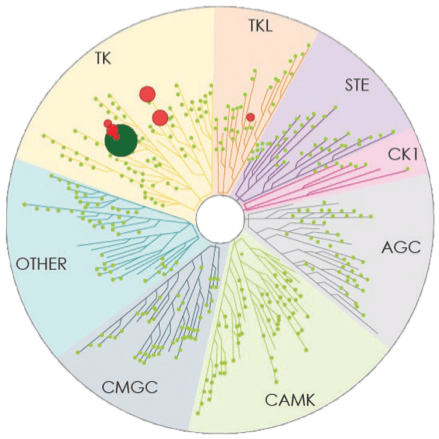
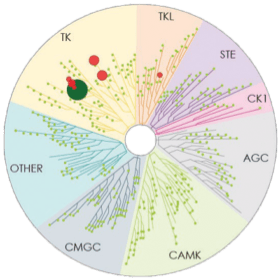
Number of kinases inhibited*: 7
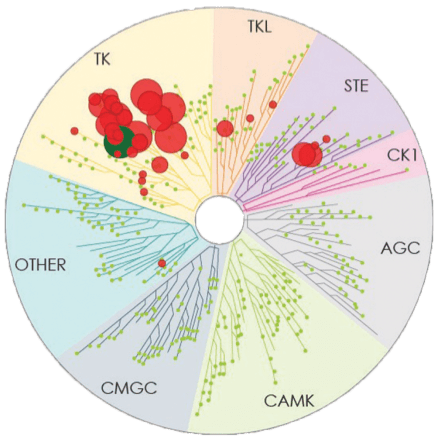
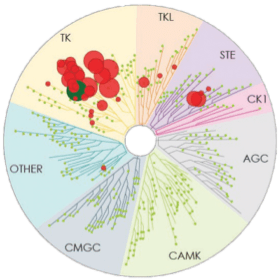
Number of kinases inhibited*: 37
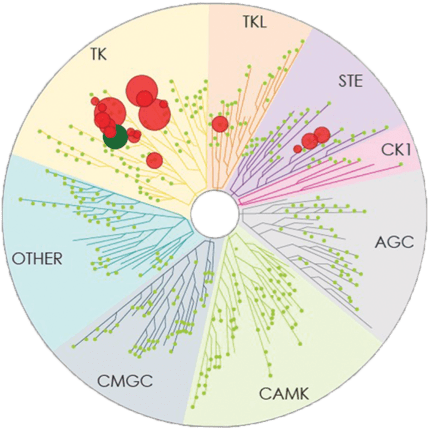

Number of kinases inhibited*: 19


- The image above displays results from an active site competitive binding assay implicating kinase inhibition. A total of 395 non-mutant kinases were screened in the testing of CALQUENCE and ibrutinib (conducted in 2014). Compounds were tested at a single concentration of 1 μM3
- Testing of zanubrutinib (conducted in 2017) included 8 additional non-mutant kinases (total of 403 screened); the 8 additional kinases were not inhibited by >65% by zanubrutinib3
- In the TREEspotTM interaction maps developed by KINOMEscan®, some circles are overlapping and therefore counting may be difficult. The degree of inhibition vs untreated control is represented by circle size2,3
- The clinical relevance of these preclinical data has not been determined. Mechanism of action statements are not meant to imply efficacy and safety
- There are no head-to-head trials between CALQUENCE and zanubrutinib comparing safety or efficacy
CALQUENCE: A highly selective next-generation BTKi that delivers focused kinase inhibition and limited off-target enzyme inhibition1-3
Dosage & Administration
Continuous BTK inhibition with
one tablet taken orally twice daily†4
Patient Financial Support
Learn about financial assistance
options for your patients
*Number of kinases inhibited >65% at a single dose (1 μM), using KINOMEscan®.2
†Approximately every 12 hours.2
BTKi=Bruton tyrosine kinase inhibitor; R/R=relapsed/refractory.
- Bond DA, Woyach JA. Targeting BTK in CLL: beyond ibrutinib. Curr Hematol Malig Rep. 2019;14(3):197-205.
- Barf T, Covey T, Izumi R, et al. Acalabrutinib (ACP-196): a covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther. 2017;363(2):240-252.
- Podoll T, Pearson PG, Kaptein A, et al. Identification and characterization of ACP-5862, the major circulating active metabolite of acalabrutinib: both are potent and selective covalent bruton tyrosine kinase inhibitors. J Pharmacol Exp Ther. 2023;384(1):173-186.
- KINOMEscan® Technology Platform. DiscoverX website. Accessed April 19, 2023. https://discoverx.com/services/drug-discovery-development-services/kinase-profiling/kinomescan
- CALQUENCE® (acalabrutinib) tablets [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2024.
You are now leaving calquencehcp.com
This link will take you to a site maintained by a third party who is solely responsible for its content. AstraZeneca provides this link as a service to website visitors. AstraZeneca is not responsible for the privacy policy of any third-party websites. We encourage you to read the privacy policy of every website you visit.
INDICATIONS AND IMPORTANT SAFETY INFORMATION
INDICATIONS AND USAGE
CALQUENCE is a Bruton tyrosine kinase (BTK) inhibitor indicated for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.
This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
CALQUENCE is also indicated for the treatment of adult patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
IMPORTANT SAFETY INFORMATION ABOUT CALQUENCE® (acalabrutinib) tablets
Serious and Opportunistic Infections
Fatal and serious infections, including opportunistic infections, have occurred in patients with hematologic malignancies treated with CALQUENCE.
Serious or Grade 3 or higher infections (bacterial, viral, or fungal) occurred in 19% of 1029 patients
Fatal and serious infections, including opportunistic infections, have occurred in patients with hematologic malignancies treated with CALQUENCE.
Serious or Grade 3 or higher infections (bacterial, viral, or fungal) occurred in 19% of 1029 patients exposed to
Serious and Opportunistic Infections
Fatal and serious infections, including opportunistic infections, have occurred in patients with hematologic malignancies treated with CALQUENCE.
Serious or Grade 3 or higher infections (bacterial, viral, or fungal) occurred in 19% of 1029 patients exposed to CALQUENCE in clinical trials, most often due to respiratory tract infections (11% of all patients, including pneumonia in 6%). These infections predominantly occurred in the absence of Grade 3 or 4 neutropenia, with neutropenic infection reported in 1.9% of all patients. Opportunistic infections in recipients of CALQUENCE have included, but are not limited to, hepatitis B virus reactivation, fungal pneumonia, Pneumocystis jirovecii pneumonia, Epstein-Barr virus reactivation, cytomegalovirus, and progressive multifocal leukoencephalopathy (PML). Consider prophylaxis in patients who are at increased risk for opportunistic infections. Monitor patients for signs and symptoms of infection and treat promptly.
Hemorrhage
Fatal and serious hemorrhagic events have occurred in patients with hematologic malignancies treated with CALQUENCE. Major hemorrhage (serious or Grade 3 or higher bleeding or any central nervous system bleeding) occurred in 3.0% of patients, with fatal hemorrhage occurring in 0.1% of 1029 patients exposed to CALQUENCE in clinical trials. Bleeding events of any grade, excluding bruising and petechiae, occurred in 22% of patients.
Use of antithrombotic agents concomitantly with CALQUENCE may further increase the risk of hemorrhage. In clinical trials, major hemorrhage occurred in 2.7% of patients taking CALQUENCE without antithrombotic agents and 3.6% of patients taking CALQUENCE with antithrombotic agents. Consider the risks and benefits of antithrombotic agents when co-administered with CALQUENCE. Monitor patients for signs of bleeding.
Consider the benefit-risk of withholding CALQUENCE for 3-7 days pre- and post-surgery depending upon the type of surgery and the risk of bleeding.
Cytopenias
Grade 3 or 4 cytopenias, including neutropenia (23%), anemia (8%), thrombocytopenia (7%), and lymphopenia (7%), developed in patients with hematologic malignancies treated with CALQUENCE. Grade 4 neutropenia developed in 12% of patients. Monitor complete blood counts regularly during treatment. Interrupt treatment, reduce the dose, or discontinue treatment as warranted.
Second Primary Malignancies
Second primary malignancies, including skin cancers and other solid tumors, occurred in 12% of 1029 patients exposed to CALQUENCE in clinical trials. The most frequent second primary malignancy was skin cancer, reported in 6% of patients. Monitor patients for skin cancers and advise protection from sun exposure.
Cardiac Arrhythmias
Serious cardiac arrhythmias have occurred in patients treated with CALQUENCE. Grade 3 atrial fibrillation or flutter occurred in 1.1% of 1029 patients treated with CALQUENCE, with all grades of atrial fibrillation or flutter reported in 4.1% of all patients. Grade 3 or higher ventricular arrhythmia events were reported in 0.9% of patients. The risk may be increased in patients with cardiac risk factors, hypertension, previous arrhythmias, and acute infection. Monitor for symptoms of arrhythmia (eg, palpitations, dizziness, syncope, dyspnea) and manage as appropriate.
Hepatotoxicity, Including Drug-Induced Liver Injury
Hepatotoxicity, including severe, life-threatening, and potentially fatal cases of drug-induced liver injury (DILI), has occurred in patients treated with Bruton tyrosine kinase inhibitors, including CALQUENCE.
Evaluate bilirubin and transaminases at baseline and throughout treatment with CALQUENCE. For patients who develop abnormal liver tests after CALQUENCE, monitor more frequently for liver test abnormalities and clinical signs and symptoms of hepatic toxicity. If DILI is suspected, withhold CALQUENCE. Upon confirmation of DILI, discontinue CALQUENCE.
ADVERSE REACTIONS
The most common adverse reactions (≥20%) of any grade in patients with relapsed or refractory MCL were anemia,* thrombocytopenia,* headache (39%), neutropenia,* diarrhea (31%), fatigue (28%), myalgia (21%), and bruising (21%). The most common Grade ≥3 non-hematological adverse reaction (reported in at least 2% of patients) was diarrhea (3.2%).
*Treatment-emergent decreases (all grades) of hemoglobin (46%), platelets (44%), and neutrophils (36%) were based on laboratory measurements and adverse reactions.
Dose reductions or discontinuations due to any adverse reaction were reported in 1.6% and 6.5% of patients, respectively. Increases in creatinine to 1.5 to 3 times the upper limit of normal (ULN) occurred in 4.8% of patients.
The most common adverse reactions (≥30%) of any grade in patients with CLL were anemia,* neutropenia,* thrombocytopenia,* headache, upper respiratory tract infection, and diarrhea.
*Treatment-emergent decreases (all grades) of hemoglobin, platelets, and neutrophils were based on laboratory measurements and adverse reactions.
In patients with previously untreated CLL exposed to CALQUENCE, fatal adverse reactions that occurred in the absence of disease progression and with onset within 30 days of the last study treatment were reported in 2% for each treatment arm, most often from infection. Serious adverse reactions were reported in 39% of patients in the CALQUENCE plus obinutuzumab arm and 32% in the CALQUENCE monotherapy arm, most often due to events of pneumonia (7% and 2.8%, respectively).
Adverse reactions led to CALQUENCE dose reduction in 7% and 4% of patients in the CALQUENCE plus obinutuzumab arm (N=178) and CALQUENCE monotherapy arm (N=179), respectively. Adverse events led to discontinuation in 11% and 10% of patients, respectively. Increases in creatinine to 1.5 to 3 times ULN occurred in 3.9% and 2.8% of patients in the CALQUENCE combination arm and monotherapy arm, respectively.
In patients with relapsed/refractory CLL exposed to CALQUENCE, serious adverse reactions occurred in 29% of patients. Serious adverse reactions in >5% of patients who received CALQUENCE included lower respiratory tract infection (6%). Fatal adverse reactions within 30 days of the last dose of CALQUENCE occurred in 2.6% of patients, including from second primary malignancies and infection.
Adverse reactions led to CALQUENCE dose reduction in 3.9% of patients (N=154), dose interruptions in 34% of patients, most often due to respiratory tract infections followed by neutropenia, and discontinuation in 10% of patients, most frequently due to second primary malignancies followed by infection. Increases in creatinine to 1.5 to 3 times ULN occurred in 1.3% of patients who received CALQUENCE.
DRUG INTERACTIONS
Strong CYP3A Inhibitors: Avoid co-administration of CALQUENCE with a strong CYP3A inhibitor. If these inhibitors will be used short-term, interrupt CALQUENCE. After discontinuation of strong CYP3A inhibitor for at least 24 hours, resume previous dosage of CALQUENCE.
Moderate CYP3A Inhibitors: Reduce the dosage of CALQUENCE to 100 mg once daily when co-administered with a moderate CYP3A inhibitor.
Strong CYP3A Inducers: Avoid co-administration of CALQUENCE with a strong CYP3A inducer. If co-administration is unavoidable, increase the dosage of CALQUENCE to 200 mg approximately every 12 hours.
SPECIFIC POPULATIONS
Based on findings in animals, CALQUENCE may cause fetal harm and dystocia when administered to a pregnant woman. There are no available data in pregnant women to inform the drug-associated risk. Advise pregnant women of the potential risk to a fetus.
Pregnancy testing is recommended for females of reproductive potential prior to initiating CALQUENCE therapy. Advise female patients of reproductive potential to use effective contraception during treatment with CALQUENCE and for 1 week following the last dose of CALQUENCE.
It is not known if CALQUENCE is present in human milk. Advise lactating women not to breastfeed while taking CALQUENCE and for 2 weeks after the last dose.
Avoid use of CALQUENCE in patients with severe hepatic impairment (Child-Pugh class C). No dosage adjustment of CALQUENCE is recommended in patients with mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment.
Please see full Prescribing Information, including Patient Information.
You may report side effects related to AstraZeneca products.
INDICATIONS AND IMPORTANT SAFETY INFORMATION
INDICATIONS AND USAGE
CALQUENCE is a Bruton tyrosine kinase (BTK) inhibitor indicated for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.
This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
CALQUENCE is also indicated for the treatment of adult patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
IMPORTANT SAFETY INFORMATION ABOUT CALQUENCE® (acalabrutinib) tablets
Serious and Opportunistic Infections
Fatal and serious infections, including opportunistic infections, have occurred in patients with hematologic malignancies treated with CALQUENCE.
Serious or Grade 3 or higher infections (bacterial, viral, or fungal) occurred in 19% of 1029 patients
Fatal and serious infections, including opportunistic infections, have occurred in patients with hematologic malignancies treated with CALQUENCE.
Serious or Grade 3 or higher infections (bacterial, viral, or fungal) occurred in 19% of 1029 patients exposed to
Serious and Opportunistic Infections
Fatal and serious infections, including opportunistic infections, have occurred in patients with hematologic malignancies treated with CALQUENCE.
Serious or Grade 3 or higher infections (bacterial, viral, or fungal) occurred in 19% of 1029 patients exposed to CALQUENCE in clinical trials, most often due to respiratory tract infections (11% of all patients, including pneumonia in 6%). These infections predominantly occurred in the absence of Grade 3 or 4 neutropenia, with neutropenic infection reported in 1.9% of all patients. Opportunistic infections in recipients of CALQUENCE have included, but are not limited to, hepatitis B virus reactivation, fungal pneumonia, Pneumocystis jirovecii pneumonia, Epstein-Barr virus reactivation, cytomegalovirus, and progressive multifocal leukoencephalopathy (PML). Consider prophylaxis in patients who are at increased risk for opportunistic infections. Monitor patients for signs and symptoms of infection and treat promptly.
Hemorrhage
Fatal and serious hemorrhagic events have occurred in patients with hematologic malignancies treated with CALQUENCE. Major hemorrhage (serious or Grade 3 or higher bleeding or any central nervous system bleeding) occurred in 3.0% of patients, with fatal hemorrhage occurring in 0.1% of 1029 patients exposed to CALQUENCE in clinical trials. Bleeding events of any grade, excluding bruising and petechiae, occurred in 22% of patients.
Use of antithrombotic agents concomitantly with CALQUENCE may further increase the risk of hemorrhage. In clinical trials, major hemorrhage occurred in 2.7% of patients taking CALQUENCE without antithrombotic agents and 3.6% of patients taking CALQUENCE with antithrombotic agents. Consider the risks and benefits of antithrombotic agents when co-administered with CALQUENCE. Monitor patients for signs of bleeding.
Consider the benefit-risk of withholding CALQUENCE for 3-7 days pre- and post-surgery depending upon the type of surgery and the risk of bleeding.
Cytopenias
Grade 3 or 4 cytopenias, including neutropenia (23%), anemia (8%), thrombocytopenia (7%), and lymphopenia (7%), developed in patients with hematologic malignancies treated with CALQUENCE. Grade 4 neutropenia developed in 12% of patients. Monitor complete blood counts regularly during treatment. Interrupt treatment, reduce the dose, or discontinue treatment as warranted.
Second Primary Malignancies
Second primary malignancies, including skin cancers and other solid tumors, occurred in 12% of 1029 patients exposed to CALQUENCE in clinical trials. The most frequent second primary malignancy was skin cancer, reported in 6% of patients. Monitor patients for skin cancers and advise protection from sun exposure.
Cardiac Arrhythmias
Serious cardiac arrhythmias have occurred in patients treated with CALQUENCE. Grade 3 atrial fibrillation or flutter occurred in 1.1% of 1029 patients treated with CALQUENCE, with all grades of atrial fibrillation or flutter reported in 4.1% of all patients. Grade 3 or higher ventricular arrhythmia events were reported in 0.9% of patients. The risk may be increased in patients with cardiac risk factors, hypertension, previous arrhythmias, and acute infection. Monitor for symptoms of arrhythmia (eg, palpitations, dizziness, syncope, dyspnea) and manage as appropriate.
Hepatotoxicity, Including Drug-Induced Liver Injury
Hepatotoxicity, including severe, life-threatening, and potentially fatal cases of drug-induced liver injury (DILI), has occurred in patients treated with Bruton tyrosine kinase inhibitors, including CALQUENCE.
Evaluate bilirubin and transaminases at baseline and throughout treatment with CALQUENCE. For patients who develop abnormal liver tests after CALQUENCE, monitor more frequently for liver test abnormalities and clinical signs and symptoms of hepatic toxicity. If DILI is suspected, withhold CALQUENCE. Upon confirmation of DILI, discontinue CALQUENCE.
ADVERSE REACTIONS
The most common adverse reactions (≥20%) of any grade in patients with relapsed or refractory MCL were anemia,* thrombocytopenia,* headache (39%), neutropenia,* diarrhea (31%), fatigue (28%), myalgia (21%), and bruising (21%). The most common Grade ≥3 non-hematological adverse reaction (reported in at least 2% of patients) was diarrhea (3.2%).
*Treatment-emergent decreases (all grades) of hemoglobin (46%), platelets (44%), and neutrophils (36%) were based on laboratory measurements and adverse reactions.
Dose reductions or discontinuations due to any adverse reaction were reported in 1.6% and 6.5% of patients, respectively. Increases in creatinine to 1.5 to 3 times the upper limit of normal (ULN) occurred in 4.8% of patients.
The most common adverse reactions (≥30%) of any grade in patients with CLL were anemia,* neutropenia,* thrombocytopenia,* headache, upper respiratory tract infection, and diarrhea.
*Treatment-emergent decreases (all grades) of hemoglobin, platelets, and neutrophils were based on laboratory measurements and adverse reactions.
In patients with previously untreated CLL exposed to CALQUENCE, fatal adverse reactions that occurred in the absence of disease progression and with onset within 30 days of the last study treatment were reported in 2% for each treatment arm, most often from infection. Serious adverse reactions were reported in 39% of patients in the CALQUENCE plus obinutuzumab arm and 32% in the CALQUENCE monotherapy arm, most often due to events of pneumonia (7% and 2.8%, respectively).
Adverse reactions led to CALQUENCE dose reduction in 7% and 4% of patients in the CALQUENCE plus obinutuzumab arm (N=178) and CALQUENCE monotherapy arm (N=179), respectively. Adverse events led to discontinuation in 11% and 10% of patients, respectively. Increases in creatinine to 1.5 to 3 times ULN occurred in 3.9% and 2.8% of patients in the CALQUENCE combination arm and monotherapy arm, respectively.
In patients with relapsed/refractory CLL exposed to CALQUENCE, serious adverse reactions occurred in 29% of patients. Serious adverse reactions in >5% of patients who received CALQUENCE included lower respiratory tract infection (6%). Fatal adverse reactions within 30 days of the last dose of CALQUENCE occurred in 2.6% of patients, including from second primary malignancies and infection.
Adverse reactions led to CALQUENCE dose reduction in 3.9% of patients (N=154), dose interruptions in 34% of patients, most often due to respiratory tract infections followed by neutropenia, and discontinuation in 10% of patients, most frequently due to second primary malignancies followed by infection. Increases in creatinine to 1.5 to 3 times ULN occurred in 1.3% of patients who received CALQUENCE.
DRUG INTERACTIONS
Strong CYP3A Inhibitors: Avoid co-administration of CALQUENCE with a strong CYP3A inhibitor. If these inhibitors will be used short-term, interrupt CALQUENCE. After discontinuation of strong CYP3A inhibitor for at least 24 hours, resume previous dosage of CALQUENCE.
Moderate CYP3A Inhibitors: Reduce the dosage of CALQUENCE to 100 mg once daily when co-administered with a moderate CYP3A inhibitor.
Strong CYP3A Inducers: Avoid co-administration of CALQUENCE with a strong CYP3A inducer. If co-administration is unavoidable, increase the dosage of CALQUENCE to 200 mg approximately every 12 hours.
SPECIFIC POPULATIONS
Based on findings in animals, CALQUENCE may cause fetal harm and dystocia when administered to a pregnant woman. There are no available data in pregnant women to inform the drug-associated risk. Advise pregnant women of the potential risk to a fetus.
Pregnancy testing is recommended for females of reproductive potential prior to initiating CALQUENCE therapy. Advise female patients of reproductive potential to use effective contraception during treatment with CALQUENCE and for 1 week following the last dose of CALQUENCE.
It is not known if CALQUENCE is present in human milk. Advise lactating women not to breastfeed while taking CALQUENCE and for 2 weeks after the last dose.
Avoid use of CALQUENCE in patients with severe hepatic impairment (Child-Pugh class C). No dosage adjustment of CALQUENCE is recommended in patients with mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment.
Please see full Prescribing Information, including Patient Information.
You may report side effects related to AstraZeneca products.
