~5-year efficacy data2
PFS risk reduction†
with CALQUENCE + obinutuzumab‡ vs GClb
The 74.5-month median follow-up data from ELEVATE-TN are not in the Prescribing Information for CALQUENCE. The timing for long-term follow-up was not prespecified, and the analysis was descriptive in nature.
- Proven efficacy as both a combination therapy and monotherapy2
- PFS benefit consistent across subgroups, including in high-risk patients2
†Based on Cox proportional-hazards model stratified by del(17p) status (yes vs no based on interactive voice/web response system).2
‡Based on log-rank test stratified by del(17p) status (yes vs no based on interactive voice/web response system).2
§Estimated 60-month OS rate in ELEVATE-TN. Median OS not reached in any arm. OS was a secondary endpoint and OS data were immature at 58.2-month median follow-up.2
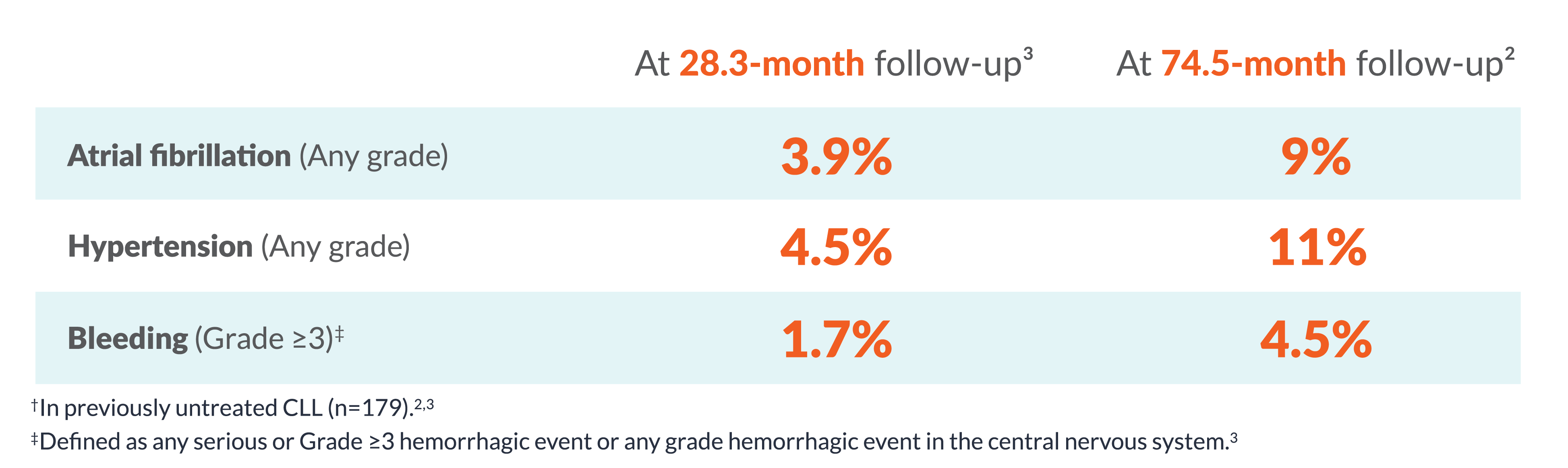
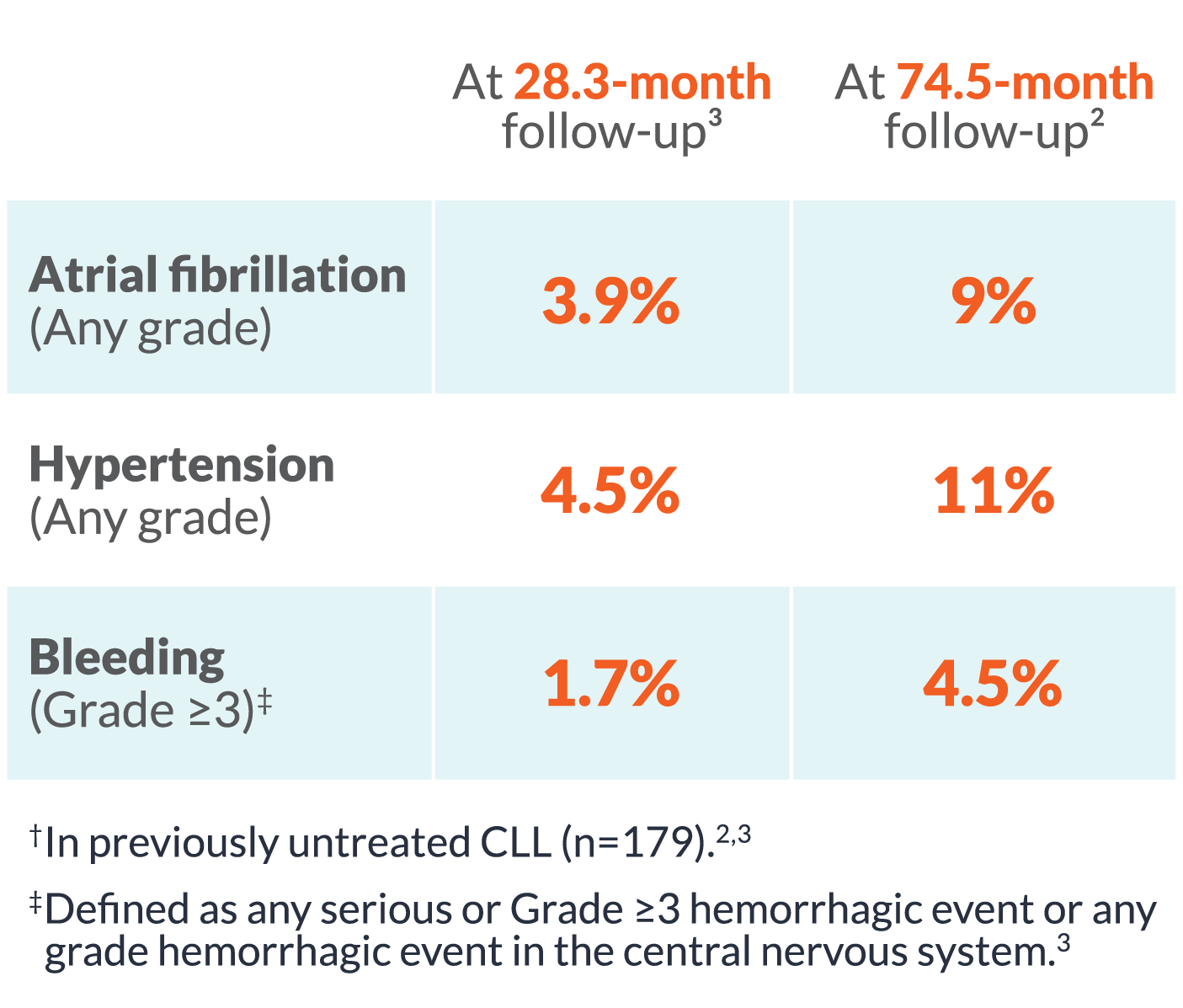
| At 28.3 month follow-up3 |
At 58.2 month follow-up2 |
|
|---|---|---|
| Atrial fibrillation (Any grade) |
4% | 7% |
| Hypertension (Any grade) |
5% | 9% |
| Bleeding (Grade ≥3) |
2% | 3% |
§In previously untreated CLL (n=179 in ELEVATE-TN).2,3
ELEVATE-TN: Safety and tolerability results at 58.2-month median follow-up were consistent with the interim analysis. The most common AEs (≥30%, any grade) in the CALQUENCE + obinutuzumab arm (n=178) were infection (79%), bleeding (49%), diarrhea (43%), headache (40%), arthralgia (34%), and neutropenia (34%). The most common AEs (≥30%, any grade) in the CALQUENCE monotherapy arm (n=179) were infection (75%), bleeding (44%), diarrhea (43%), and headache (39%).2
ELEVATE-RR: At 41-month median follow-up, the most common AEs (≥20%, any grade) in patients receiving CALQUENCE (n=266)/ibrutinib (n=263) were infections (78%/81%), bleeding (38%/51%), diarrhea (35%/46%), headache (35%/20%), cough (29%/21%), cardiac events (24%/30%), pyrexia (23%/19%), anemia (22%/19%), neutropenia (21%/25%), fatigue (20%/17%), arthralgia (16%/23%), and hypertension (9%/23%).3
ASCEND: Safety and tolerability results at 46.5-month median follow-up were consistent with the interim analysis. The most common AEs (≥20%, any grade) in patients receiving CALQUENCE (n=154) were infection (68%), hemorrhage (31%), neutropenia (24%), headache (23%), and diarrhea (21%).4
At 28.3-month median follow-up in ELEVATE-TN, the most common ARs (≥30%) of any grade in patients treated with CALQUENCE + obinutuzumab (n=178) were infection (69%), neutropenia (53%), anemia (52%), thrombocytopenia (51%), headache (40%), diarrhea (39%), musculoskeletal pain (37%), fatigue (34%), and bruising (31%). In the CALQUENCE monotherapy arm (n=179), the most common ARs (≥30%) of any grade were infection (65%), anemia (53%), headache (39%), diarrhea (35%), musculoskeletal pain (32%), and thrombocytopenia (32%).1
Safety and tolerability at 74.5-month median follow-up were consistent with the interim analysis, apart from COVID-19, which was observed during the pandemic.2
The 74.5-month median follow-up data from ELEVATE-TN are not in the Prescribing Information for CALQUENCE.
In a pooled analysis of 1029 patients with CALQUENCE, Grade 3 or higher ventricular arrhythmia events were reported in 0.9% of patients.
||Events inclusive of preferred group terms and are not limited to cardiovascular body system.2-4
¶58.2-month median follow-up (CALQUENCE + obinutuzumab; n=178 and CALQUENCE monotherapy; n=179) in the ELEVATE-TN trial.2
#41-month median follow-up (CALQUENCE; n=266 and ibrutinib; n=263) in the ELEVATE-RR trial.3
**Grade ≥3 atrial fibrillation/flutter events were 4.9% for CALQUENCE and 3.8% for ibrutinib.3
††46.5-month median follow-up (CALQUENCE; n=154) in the ASCEND trial.4
Studied in multiple Phase 3 trials in CLL3-5
ELEVATE-TN: CALQUENCE ± G vs GClbPREVIOUSLY UNTREATED CLL
The first Phase 3 study of a BTKi
with and without obinutuzumab
vs chemoimmunotherapy3
ELEVATE-RR: CALQUENCE vs ibrutinibRELAPSED/REFRACTORY CLL
The first Phase 3 study of a next-generation BTKi
vs ibrutinib in R/R CLL4
ASCEND: CALQUENCE vs IdR/BRRELAPSED/REFRACTORY CLL
The first Phase 3 study of a
BTKi vs investigator’s choice
of IdR or BR5
POOLED
CARDIOVASCULAR SAFETY
Analysis of select cardiovascular
adverse events with CALQUENCE
across 4 CLL
clinical trials7
Hear about Dr Danilov's experience prescribing CALQUENCE
AEs=adverse events; BR=bendamustine + rituximab; BTKi=Bruton tyrosine kinase inhibitor; CI=confidence interval; CLL=chronic lymphocytic leukemia; CV=cardiovascular; G=obinutuzumab; GClb=obinutuzumab + chlorambucil; HR=hazard ratio; IdR=idelalisib + rituximab; INV=investigator; IRC=Independent Review Committee; NCCN=National Comprehensive Cancer Network; ORR=overall response rate; OS=overall survival; PFS=progression-free survival; PPI=proton pump inhibitors; R/R=relapsed/refractory; SLL=small lymphocytic lymphoma.
- CALQUENCE® (acalabrutinib) tablets [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2024.
- Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib ± obinutuzumab vs obinutuzumab + chlorambucil in treatment-naive chronic lymphocytic leukemia: 6-year follow-up of ELEVATE-TN [abstract and presentation]. Presented at: American Society of Hematology (ASH) Annual Meeting; December 9-12, 2023. San Diego, CA. Abs 636.
- Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukemia (ELEVATE-TN): a randomised, controlled, phase 3 trial [published correction appears in Lancet. 2020;395(10238):1694]. Lancet. 2020;395(10232):1278-1291.
- Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39(31):3441-3452 and supplementary appendix.
- Ghia P, Pluta A, Wach M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2849-2861.
- Ghia P, Pluta A, Wach M, et al. Acalabrutinib versus investigator's choice in relapsed/refractory chronic lymphocytic leukemia: final ASCEND trial results. Hemasphere. 2022;6(12):e801.
- Brown JR, Byrd JC, Ghia P, et al. Cardiovascular adverse events in patients with chronic lymphocytic leukemia receiving acalabrutinib monotherapy: pooled analysis of 762 patients. Haematologica. 2022;107(6):1335-1346.
- Barf T, Covey T, Izumi R, et al. Acalabrutinib (ACP-196): a covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther. 2017;363(2):240-252.
- Podoll T, Pearson PG, Kaptein A, et al. Identification and characterization of ACP-5862, the major circulating active metabolite of acalabrutinib: both are potent and selective covalent Bruton tyrosine kinase inhibitors . J Pharmacol Exp Ther. 2023;384(1):173-186.
- Data on File, US-90733. AstraZeneca Pharmaceuticals LP.