Efficacy
The first Phase 3 study of a BTKi with and without obinutuzumab vs chemoimmunotherapy1
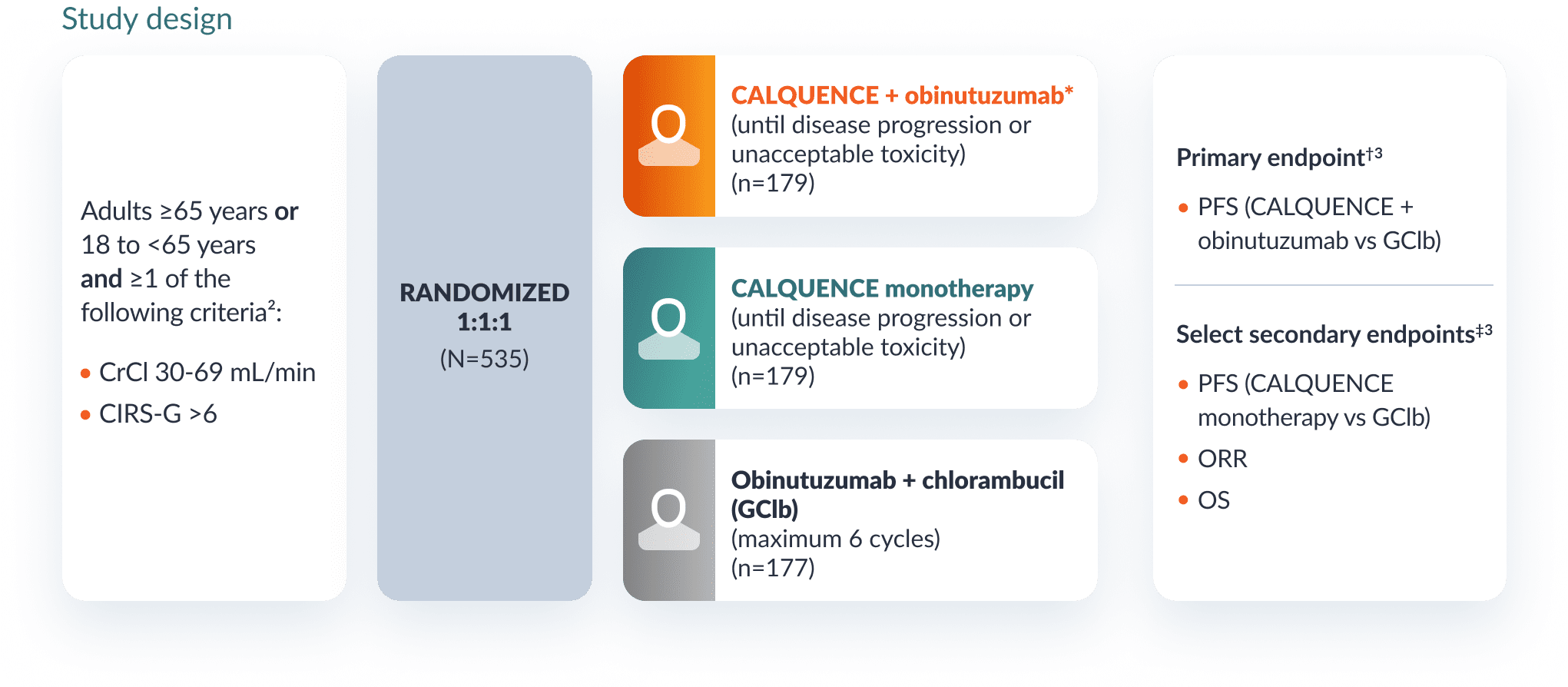
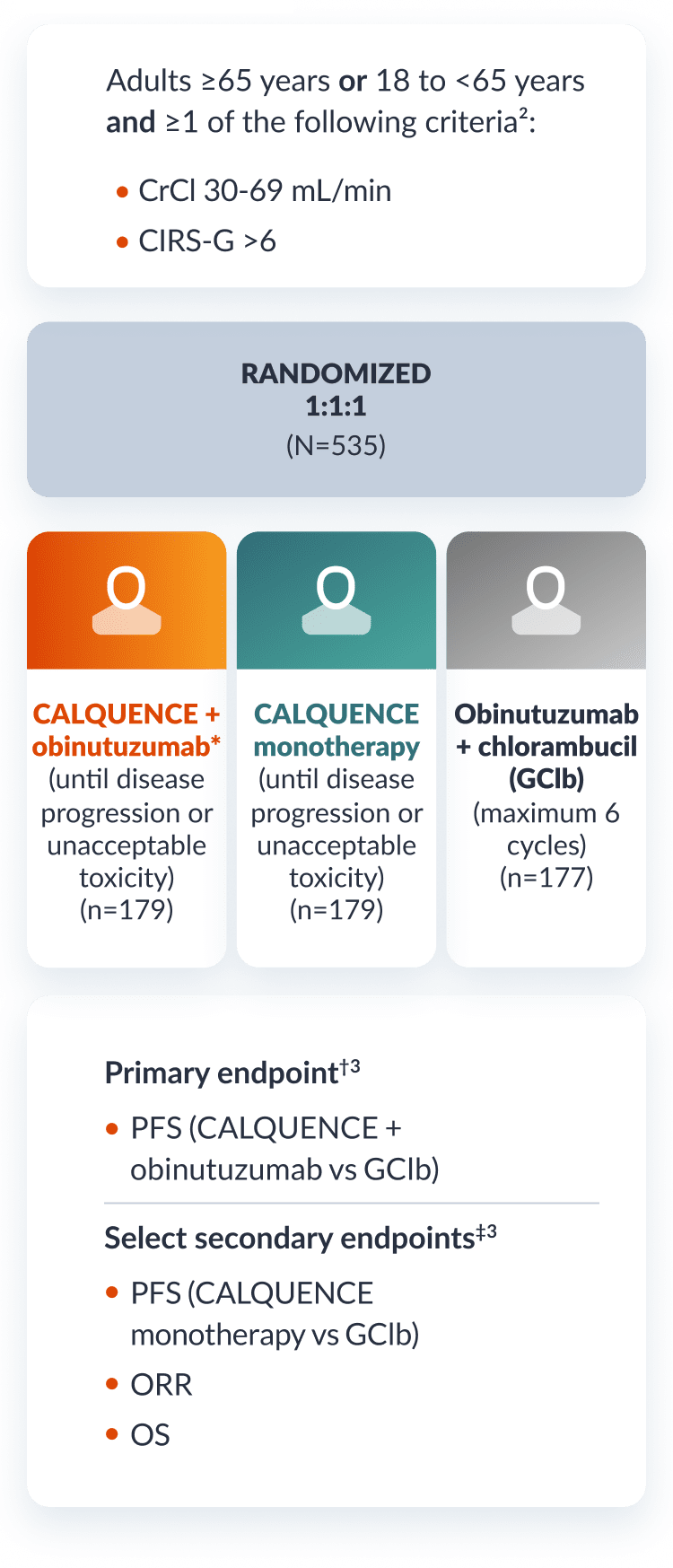
A randomized, multicenter, open-label study in patients with previously untreated CLL2
Study design
Patients received CALQUENCE 100 mg orally approximately every 12 hours until disease progression or unacceptable toxicity for either CALQUENCE + obinutuzumab or CALQUENCE monotherapy.2
Interim analysis had a median follow-up of 28.3 months (range: 0.0-40.8 months).1,2
Long-term analysis had a median follow-up of 74.5 months (range: 0.0-89.0 months).3
*For CALQUENCE + obinutuzumab, obinutuzumab was given 28 days after the first dose of CALQUENCE (Cycle 2, Day 1), and was given for up to 6 cycles. Refer to the obinutuzumab Prescribing Information for recommended obinutuzumab dosing information.2
†The primary endpoint at the interim analysis was IRC-assessed PFS (CALQUENCE + obinutuzumab vs GClb). After the interim analysis at 28.3 months, PFS was INV-assessed only.2,3
‡Select secondary endpoints at interim analysis were IRC-assessed PFS (CALQUENCE monotherapy vs GClb), IRC-assessed ORR, OS, and safety. After the interim analysis at 28.3 months, PFS and ORR were INV-assessed only.1,3
§Equivalent vitamin K antagonists were not allowed.2
BTKi=Bruton tyrosine kinase inhibitor; CIRS-G=Cumulative Illness Rating Scale–Geriatric; CLL=chronic lymphocytic leukemia; CrCl=creatinine clearance; INV=investigator; IRC=Independent Review Committee; ORR=overall response rate; OS=overall survival; PFS=progression-free survival.
- Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE-TN): a randomised, controlled, phase 3 trial [published correction appears in Lancet. 2020;395(10238):1694]. Lancet. 2020;395(10232):1278-1291 and supplementary appendix.
- CALQUENCE® (acalabrutinib) tablets [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2024.
- Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib ± obinutuzumab vs obinutuzumab + chlorambucil in treatment-naive chronic lymphocytic leukemia: 6-year follow-up of ELEVATE-TN [abstract and presentation]. Presented at: American Society of Hematology (ASH) Annual Meeting; December 9-12, 2023. San Diego, CA. Abs 636.